USPTO TRIES TO PLUG IN THE VOID
The U.S. Supreme Court has had several opportunities to provide clarity but refused to revisit the patent eligibility of diagnostic methods. For example, in 2020 alone, the Supreme Court declined to hear eight appeals of patent eligibility cases. However, even though the Federal Circuit failed to provide any clarity about subject-matter eligibility, the USPTO in 2019 stepped in to shed some much-needed light on “something more.” The USPTO’s 2019 Revised Patent Subject Matter Eligibility Guidance distinguishes between broad, general claims that recite nothing more than a judicial exception and those that are narrow or specific. 84 Fed. Reg. 50 (January 7, 2019).

The guidance significantly communicates the need to evaluate whether a judicial exception is integrated into a practical application. Id. at 54. The Office reframed the test and the question (see figure 1) of whether a claim is directed to a judicial exception, like a natural law, abstract idea, and laws of nature. They framed the test into two separate and distinct tests and questions: Does the claim recite a judicial exception? And if it does, does the claim recite additional elements that integrate the judicial exception into a practical application? The answer to the first question is relative straightforwards. The second question aims to understand whether a judicial exception is applied, relied on, or used in a manner that imposes a meaningful limitation on the judicial exception. In other words, the guidance is useful for ascertaining if the claim as a whole has integrated into it a practical application (see figure 2). This guidance, as intended, will assist Examiners in being consistent, increasing clarity and predictability, determining if a claim is or is not more than a judicial exception during patent prosecution when Section 101 is applied. Still, for litigators, the more considerable concern remains, will this impact the Federal Circuit’s interpretation of Supreme Court precedent? The answer may remain splintered, and even though courts do not typically give deference to USPTO guidance, the court decision may vary depending upon the court.
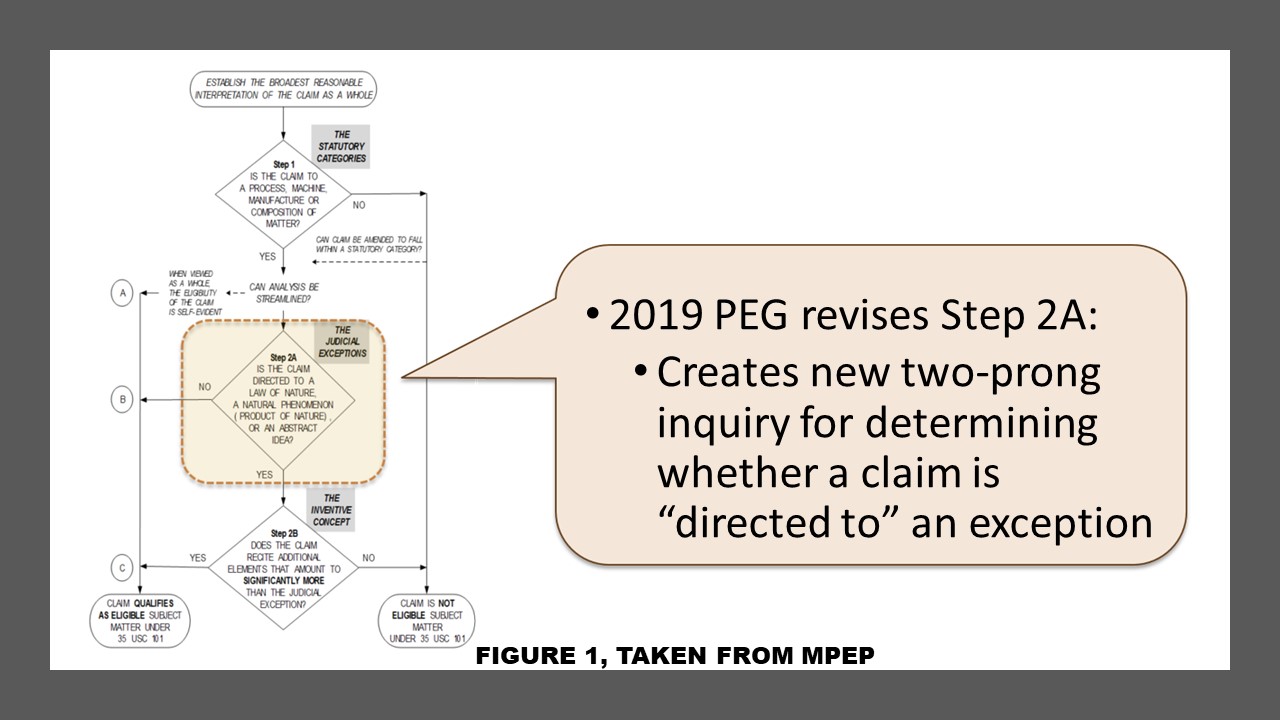
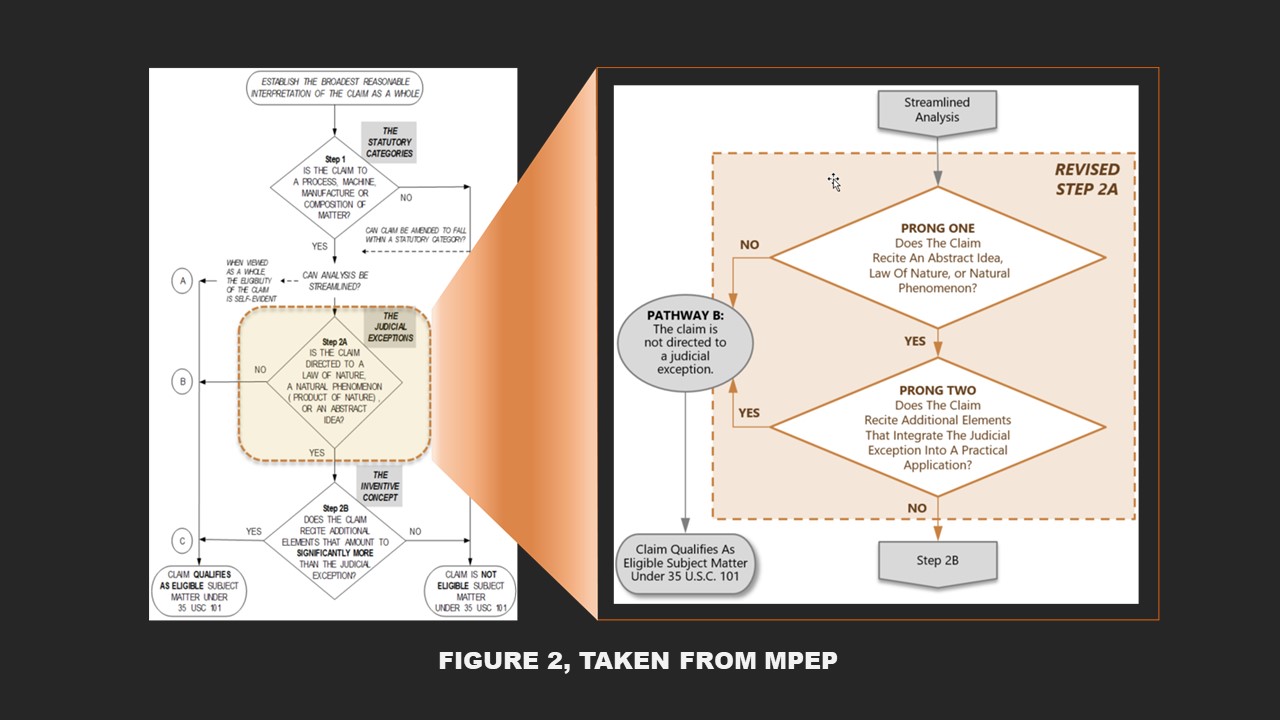
Moreover, the not well-understood, non-routine, and unconventional step of the Alice/Mayo test remains part of the new test for biotechnology, diagnostics, pharma, life-sciences subject matter eligibility. Thus, after the step for ” practical application,” the USPTO merely moved the non-routine and unconventional step to a later stage.
In the next blog, I will discuss various strategies for drafting patents for biotech/pharma companies.

